22 Mar 2021
Vaccines: An Unprecedented Race to Beat the Virus
Estimated read time: 6 minutes, 15 seconds
The COVID-19 pandemic has unleashed one of the most accelerated races in the history of science. Less than a year since infections from the novel coronavirus were first recorded, several vaccines have been approved, and the goal of putting an end to the global health, social, and economic crisis seems within reach.
Through the COVAX program, the World Health Organization (WHO) is working directly with governments, negotiating pricing and coordinating the distribution of vaccines to ensure that everyone in the world has equal access to them.
The three phases of vaccine development, from testing for safety and efficacy in laboratory rats to trials on thousands of participants, usually take years. And getting the vaccine authorized is a multi-step, bureaucratic labyrinth that makes the process even longer.
This time, scientists, pharmaceutical companies, universities, and private and public institutions achieved promising results and emergency use authorizations in record time.
Thanks to this, immunization efforts are already underway in much of the world.
The majority of approved vaccines require two doses to generate immunity. The doses are administered three to four weeks apart. So far, they have been approved for individuals 16 years of age and older.
Clinical trials for the COVID-19 vaccine in children between 12 and 15 years of age are currently underway. The vaccine may be authorized for children older than 12 by the end of May or beginning of August of this year, but shots for younger kids may not be approved until next year. The next step will be to determine whether this vaccine will be included in immunization schedules and required for school entry.
How Does a Vaccine Get Approved?

It can be interesting to learn about the process by which this incredible tool for preventing disease makes it into our arms. All vaccines, not just the COVID-19 one, must undergo testing and development phases:
Pre-Clinical Stage: Uses tissue-culture or cell-culture systems, and animal testing, to evaluate the safety of the vaccine and its ability to produce an immune response. These findings help determine its future implementation in trials involving human participants.
Phase I: The new vaccine is tested on a small group of people (usually around 100) to evaluate its safety and biological effects, including its immunogenicity, a measure of an antigen’s ability to activate the immune system.
Phase II: The goal is to study the vaccine’s safety and its immunogenicity as well as its proposed doses and delivery method. and method of delivery. A larger group of people participate in Phase II testing than in the previous phase (between 200 and 500.)
Phase III: This tends to be the step that comes before vaccine approval, and includes assessing the vaccine’s safety and efficacy in a large group of people. It involves hundreds of thousands of people from one or several countries. The tests are randomized and double blind (meaning that neither the person receiving the shot nor the person administering it know whether it contains the vaccine). The vaccine is also tested against a placebo (a harmless substance that has no therapeutic effects.)
Phase IV: These trials are conducted after a vaccine is approved in one or multiple countries. Their goal is to assess how the vaccine is working on the larger population, evaluating its efficiency and monitoring side effects.
How Do COVID-19 Vaccines Work?
So far, COVID-19 vaccines work similarly to the flu shot. They don’t prevent the virus from spreading, but if a person is exposed to the coronavirus and gets sick, the vaccine helps prevent a more severe and potentially fatal form of the disease.
The duration that immunity lasts after getting any of the vaccines remains unknown. We also don’t know whether the COVID-19 vaccine will be seasonal, like the flu shot, which people need to get every year because the virus mutates.

Additional monitoring of the vaccine is required to address these particular questions.
The pandemic has shown us that vaccine development can be accelerated and be made more efficient. But it has also paved the way for a new generation of immunizations.
Such is the case with Novavax, a new vaccine that, if approved, could be one of the most powerful tools against the coronavirus pandemic, offering several benefits over its competitors. Preliminary data shows that the Novavax shot would be one of the first to stop the asymptomatic spread of the virus and bring longer-lasting protection.
The Pfizer and Moderna vaccines are based on a new technology that injects genetic material via molecules known as messenger RNA, or mRNA. This genetic code instructs the body to create the coronavirus’s signature spike protein in order to trigger an immune response. The Novavax vaccine, on the other hand, involves injecting a synthetic and slightly modified version of the spike protein instead of instructing the body to create it.
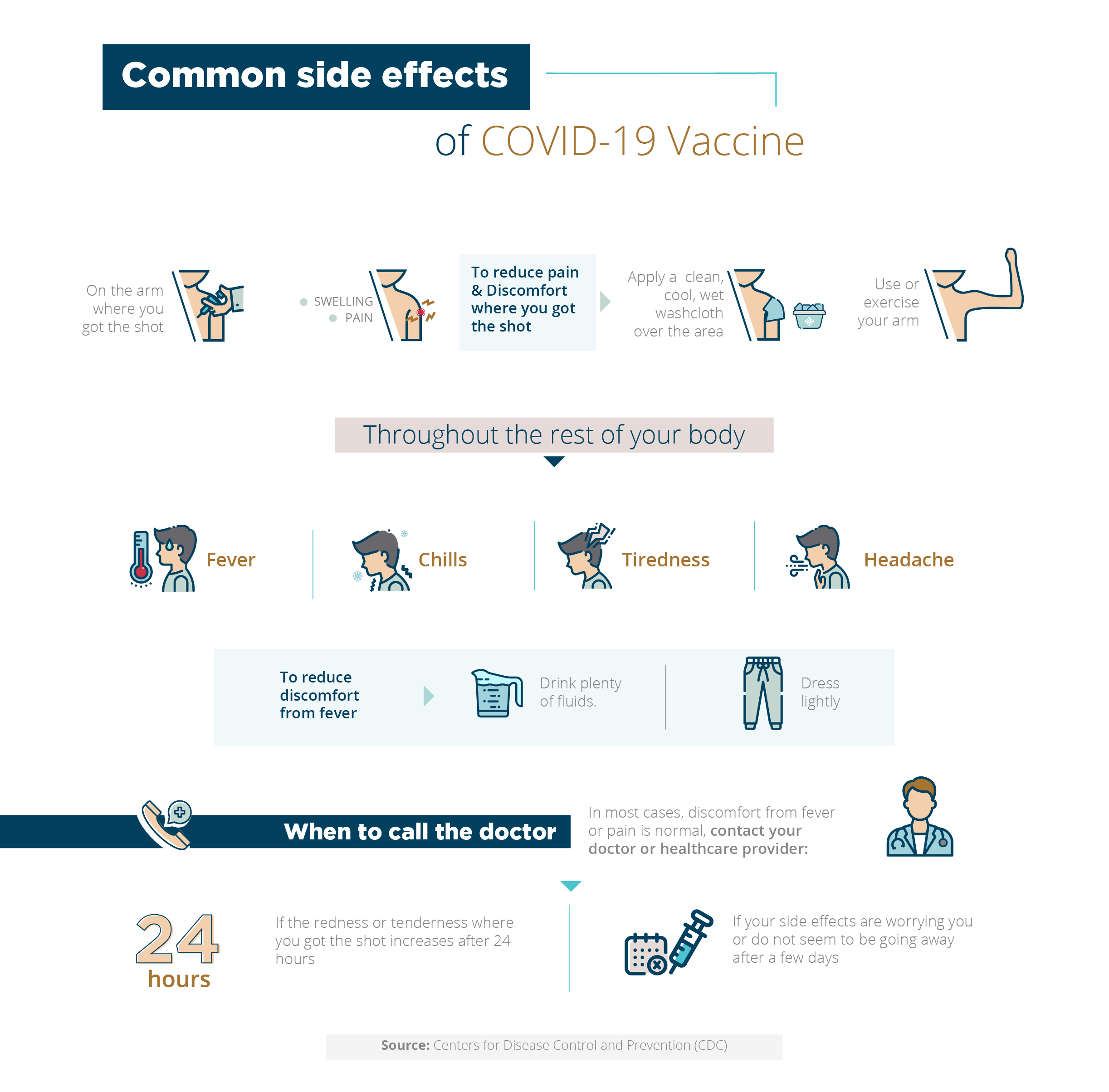
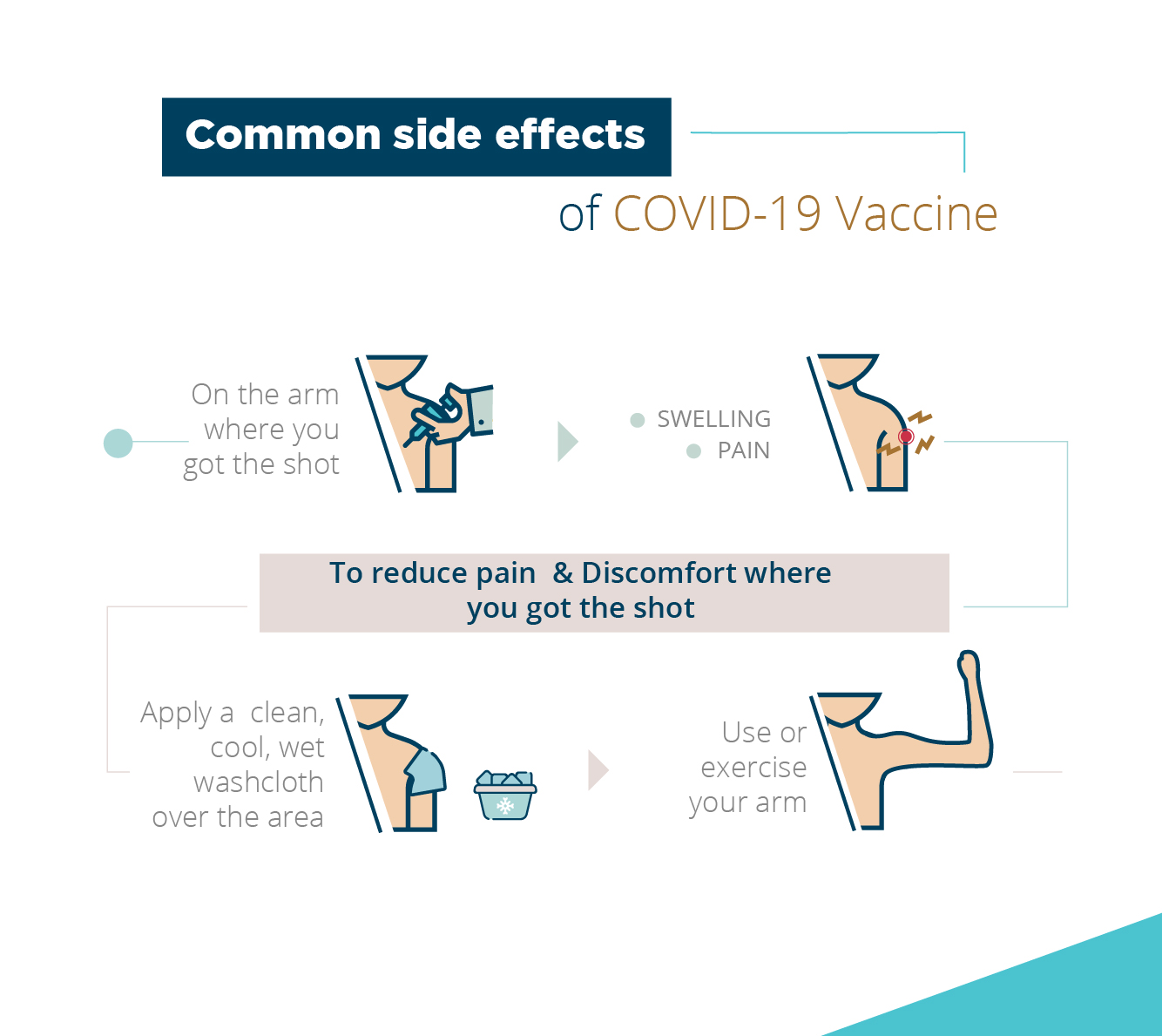
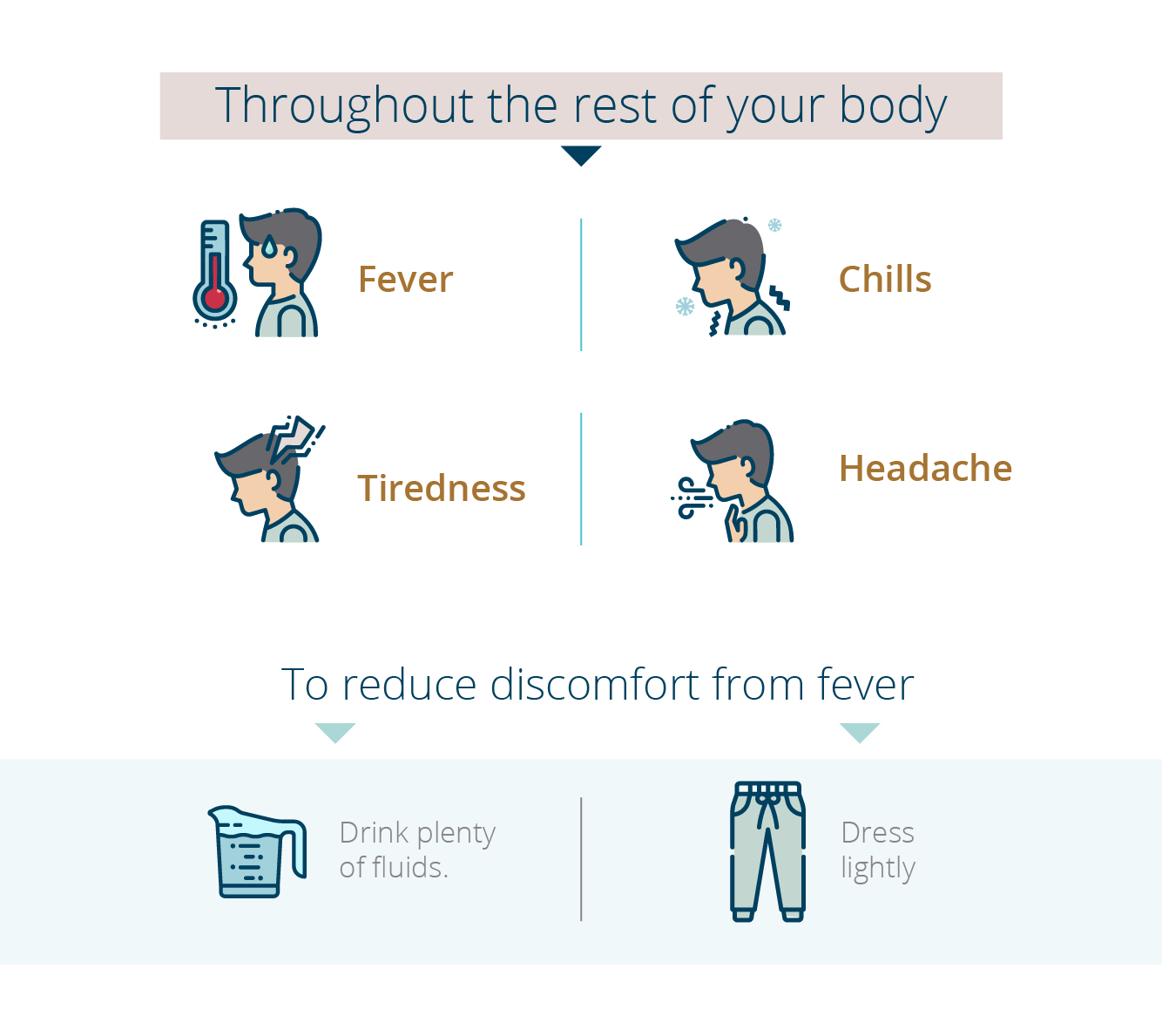
Side Effects
Approved Vaccines (February 2021)
A total of 11 COVID-19 vaccines have been approved so far. Additionally, there are 58 vaccines in different stages of development and 170 scientific studies tracking and monitoring immunization.
- Pfizer-Biotech
Vaccine Type: Uses genetic material known as mRNA to generate a genetic response.
Where It’s Used: Albania, Argentina, Australia, Bahrain, Canada, Chile, Colombia, Costa Rica, Ecuador, European Union, Greenland, Iceland, Iraq, Israel, Jordan, Kuwait, Malaysia, Mexico, New Zealand, Noruega, Oman, Panama, the Philippines, Qatar, Saudi Arabia, Serbia, Singapore, United Arab Emirates, United Kingdom, United States, Vatican City. This is also the vaccine that is being distributed by the OMS. - Moderna
Vaccine Type: Uses genetic material known as mRNA to generate a genetic response.
Where It’s Used: Canada, United States, Greenland, Iceland, Israel, Norway, Qatar, Saudi Arabia, Singapore, United Kingdom. - AstraZeneca-Oxford
Vaccine Type: Uses a weakened version of a common cold virus (known as an adenovirus).
Where It’s Used: Argentina, Bahrain, Bangladesh, Brazil, Chile, Dominican Republic, Ecuador, El Salvador, United States, Hungary, India, Iraq, Mexico, Morocco, Myanmar, Nepal, Pakistan, the Philippines, Saudi Arabia, South Africa, South Korea, Sri Lanka, Thailand, United Kingdom, Vietnam. - Sputnik (Russia, Gamaleya National Center of Epidemiology and Microbiology)
Vaccine Type: Uses a weakened version of two types of adenoviruses that cause the common cold.
Where It’s Used: Algeria, Argentina, Armenia, Bahrain, Belarus, Bolivia, Egypt, Gabon, Ghana, Guatemala, Guinea, Guyana, Honduras, Hungary, Iran, Kazakhstan, Kyrgyzstan, Lebanon, Mexico, Mongolia, Montenegro, Myanmar, Nicaragua, Pakistan, Palestine, Paraguay, Republika Srpska, Russia, Saint Vincent and the Grenadines, San Marino, Serbia, Syria, Tunisia, Turkmenistan, United Arab Emirates, Uzbekistan, Venezuela. - Johnson & Johnson
The most recently approved vaccine. It requires only one dose. Uses an inactivated version of the common cold virus to transport genetic material to the cells.
It is approved for use in the United States, Canada, and the United Kingdom. - EpiVacCorona (Russia, Federal Budgetary Institution of Science The State Research Center for Virology and Biotechnology)
Vaccine Type: Triggers an immune response using synthetic virus proteins.
Where It’s Used: Russia, Turkmenistan - CoronaVac (China, Sinovac)
Vaccine Type: Uses inactivated viruses to generate an immune response.
Where It’s Used: Azerbaijan, Bolivia, Brazil, Cambodia, China, Chile, Colombia, Hong Kong, Indonesia, Laos, Mexico, Thailand, Turkey, Philippines, Uruguay. - BBIBP-CorV (China, Beijing Institute of Biological Products; China National Pharmaceutical Group)
Vaccine Type: Uses inactivated viruses to generate an immune response.
Where It’s Used: Argentina, Bahrain, Cambodia, China, Egypt, Hungary, Iraq, Jordan, Laos, Macau, Morocco, Nepal, Pakistan, Peru, Senegal, Serbia, Seychelles, UAE, Zimbabwe - Convidicea
Vaccine Type: Uses five different kinds of recombinant adenoviruses.
Where It’s Used: Mexico, China (military use), Pakistan - Chinese Vaccine (no name announced yet)
Vaccine Type: Uses inactivated viruses to generate an immune response.
Where It’s Used: China - Covaxin (India, Bharat Biotech)
Vaccine Type: Uses inactivated viruses to generate an immune response.
Where It’s Used: India
Please note that the COVID-19 healthcare landscape frequently changes, and that this article based upon information available to date.